
To receive a COVID-19 vaccine for YOUR CHILD, please select the appropriate age group.
For children:
6 months to 4 years, click HERE.
5 – 11 years, click HERE.
12 years and older, click HERE.
Once you have completed the vaccine information and consent forms, a member of the HOPE Clinic staff will call you to schedule an appointment for your child.
Vaccines Are Widely Available for Everyone Ages Six Months and Older

Children ages six months and older can now get Pfizer-BioNTech and Moderna COVID-19 vaccines.
Booster shots are available for some people age 18 years and older who received Pfizer-BioNTech or Moderna vaccines, who are at higher risk for COVID-19 exposure or severe illness, and everyone age 18 years and older who has had one shot of J&J/Janssen.
Moderately to severely immunocompromised people ages 12 years and older who completed their Pfizer-BioNTech COVID-19 vaccine primary series and ages 18 years or older who complete their Moderna COVID-19 vaccine primary series should plan to get an additional primary dose followed by a booster dose.
Frequently Asked Questions
Q: How many doses of the COVID-19 vaccine do I need to get?
A: Refer to the chart below for information on the number of vaccine doses required:
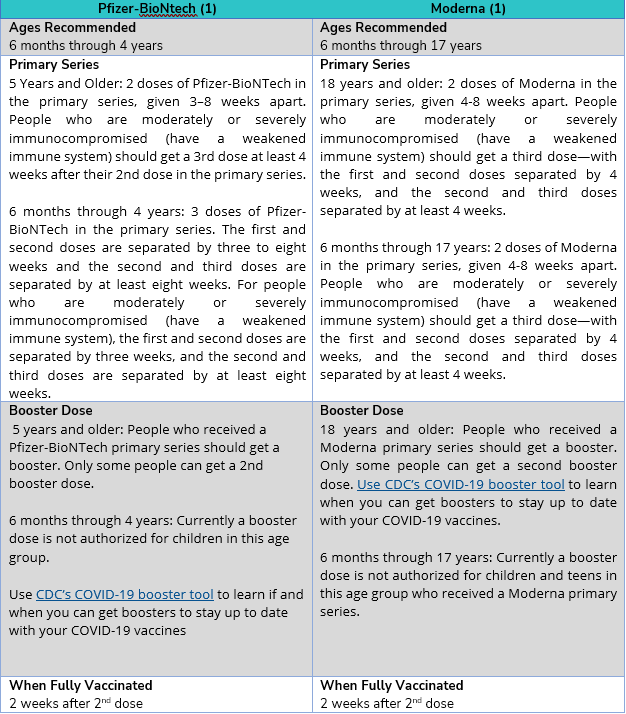
Q: Can I get COVID-19 from the vaccine?
A: No. It is not possible to get COVID-19 from vaccines. The Moderna vaccine uses only a gene from the virus while other vaccines being studied use inactivated virus. NONE of these can cause COVID-19.
Q: After getting a COVID-19 vaccine, will I test positive for COVID-19 on a viral test?
A: No. Neither the recently authorized and recommended vaccines nor the other COVID-19 vaccines currently in clinical trials in the United States can cause you to test positive on viral tests, which are used to see if you have a current infection.
If your body develops an immune response—the goal of vaccination—there is a possibility you may test positive on some antibody tests. Antibody tests indicate you had a previous infection and that you may have some level of protection against the virus. Experts are currently looking at how COVID-19 vaccination may affect antibody testing results.
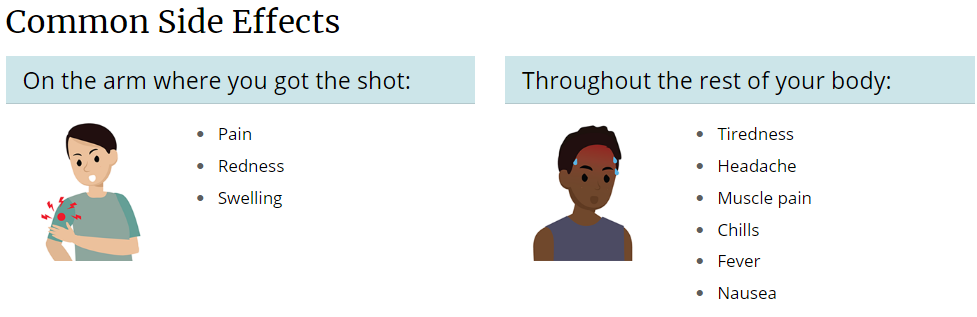
Q: What are common side effects of the COVID-19 vaccine?
A: The most commonly reported side effects, which typically lasted several days, were pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea and vomiting, and fever.
Q: How long will it take for the vaccine to protect me?
A: According to the CDC, it will take approximately one to two weeks following the 2nd dose of the Moderna vaccine to be considered fully vaccinated and protected. Full protection from the Janssen Johnson & Johnson vaccine can be expected within 2 to 4 weeks after injection.
Q: Do I have to continue wearing a mask after I get the vaccine?
A: Yes. We should continue wearing masks, practicing good hand hygiene and social distancing until enough vaccine has been manufactured and distributed to slow the spread of COVID-19. More information is also needed about how long the COVID-19 will provide antibodies.
Q: Will I be monitored for any side effects after I receive the COVID-19 vaccine?
A: Yes. Vaccine providers will monitor patients for at least 15 minutes after each dose in case of immediate adverse reactions to the vaccine. Any patient with an adverse history to injectable vaccines may be monitored longer. In addition, each patient will be encouraged to sign up with the CDC monitoring program called V-Safe. To learn more about V-Safe, click HERE.
Possible Side Effects After Getting a COVID-19 Vaccine
COVID-19 vaccination will help protect people from getting COVID-19. Adults and children may have some side effects from the vaccine, which are normal signs that their body is building protection. These side effects may affect their ability to do daily activities, but they should go away in a few days. Some people have no side effects, and allergic reactions are rare.
Serious side effects that could cause a long-term health problem are extremely unlikely following any vaccination, including COVID-19 vaccination. Vaccine monitoring has historically shown that side effects generally happen within six weeks of receiving a vaccine dose. For this reason, the U.S. Food and Drug Administration (FDA) collected data on each of the authorized COVID-19 vaccines for a minimum of two months (eight weeks) after the final dose. CDC is continuing to monitor the safety of COVID-19 vaccines even now that the vaccines are in use.
For more information from the CDC on common side effects, please click HERE.
To register for the V-Safe program AFTER you’ve received your vaccination, click the image below:
For more answers, please see the CDC page Frequently Asked Questions about COVID-19 Vaccination.
FTCA
As a Federally Qualified Health Center (FQHC), HOPE receives HHS funding and Federal Public Health Service (PHS) deemed status with respect to certain health or health-related claims, including medical malpractice claims, for itself and its covered individuals.
HOPE Clinic is a Health Center Program grantee under 42 U.S.C. 254b, and a deemed Public Health Service employee under 42 U.S.C. 233(g)-(n).




